
TROUBLESHOOTING BY PALSGAARD
TECHNICAL ARTICLE
Ice cream quality suffers from the effects of temperature fluctuations during transport and storage. Discover what lies at the root of the problem and how the right combination of emulsifiers and stabilisers can help ice cream manufacturers maintain good ice cream from the factory doors to the consumer's table.

As almost everyone in the world knows, ice cream is extremely sensitive to changes in temperature. Most would think of the effect of the sun, for example, melting their tasty treat on a summer’s day. But even relatively minor fluctuations during the long journey from the production plant to those delicious, cool mouthfuls can cause the quality of ice cream products to deteriorate. And the undesirable evidence of such temperature ups and downs includes a loss of taste, a sandy, gritty mouthfeel, and obvious water crystallization.
Of course, controlling every possible point of temperature fluctuation – in the factory, during load-out, in transit, entering the retail store, placement and storage in display cabinets, the trip to the consumer’s home and so on – is beyond any manufacturer’s capabilities. But there is much that can be done in terms of both the recipe and the process to ensure the product is ready to put up a good fight, maintaining as much as possible of its original quality.

Ice cream is a complex system of foam, containing a gas (air) dispersed as small cells in a partially frozen continuous phase. In the continuous phase, fat is dispersed as an inner phase in an emulsion, where the milk solids and stabilisers are in a colloidal solution and sugar and salts form a true solution.
Three ingredient types exert the most influence on a typical ice cream’s ability to withstand heat shock: milk solids, sugars, and additives in the form of emulsifiers and stabilisers.
Milk solids and sugars both have a lot to say when it comes to the freezing point of the ice cream, so it is important to get both the types and amounts used exactly right.
Fat, while important for the product’s structure, has little effect on its ice crystals, since it works in the fat phase and not in the water phase.
Milk solids act to stabilize ice cream and provide body for the product’s networked structure.
But they are an expensive part of the product, making their reduction a desirable move for many manufacturers. If less milk solid is used, however, there will be a greater proportion of water in the product. And all that moisture needs to be crystallized – resulting in a watery mouthfeel and greater instability.
Choosing a milk solid that can combat heat shock requires manufacturers to have a good eye for quality. First, the milk solid needs to be in a form that is best able to support the formation of the desired texture. Most often, a powder is used, but milk or cream is also relatively common.
Sugars play twin roles in ice cream: helping to control taste and the product’s freezing point. Adding a lot of sugar lowers the freezing point, helping to achieve the softness needed, for example, for scoopable ice cream applications. But doing so also makes the product more vulnerable to damage along its journey, with more free water moving to attach to the existing ice crystals and causing them to grow. Too little sugar, on the other hand, makes the ice cream harder.
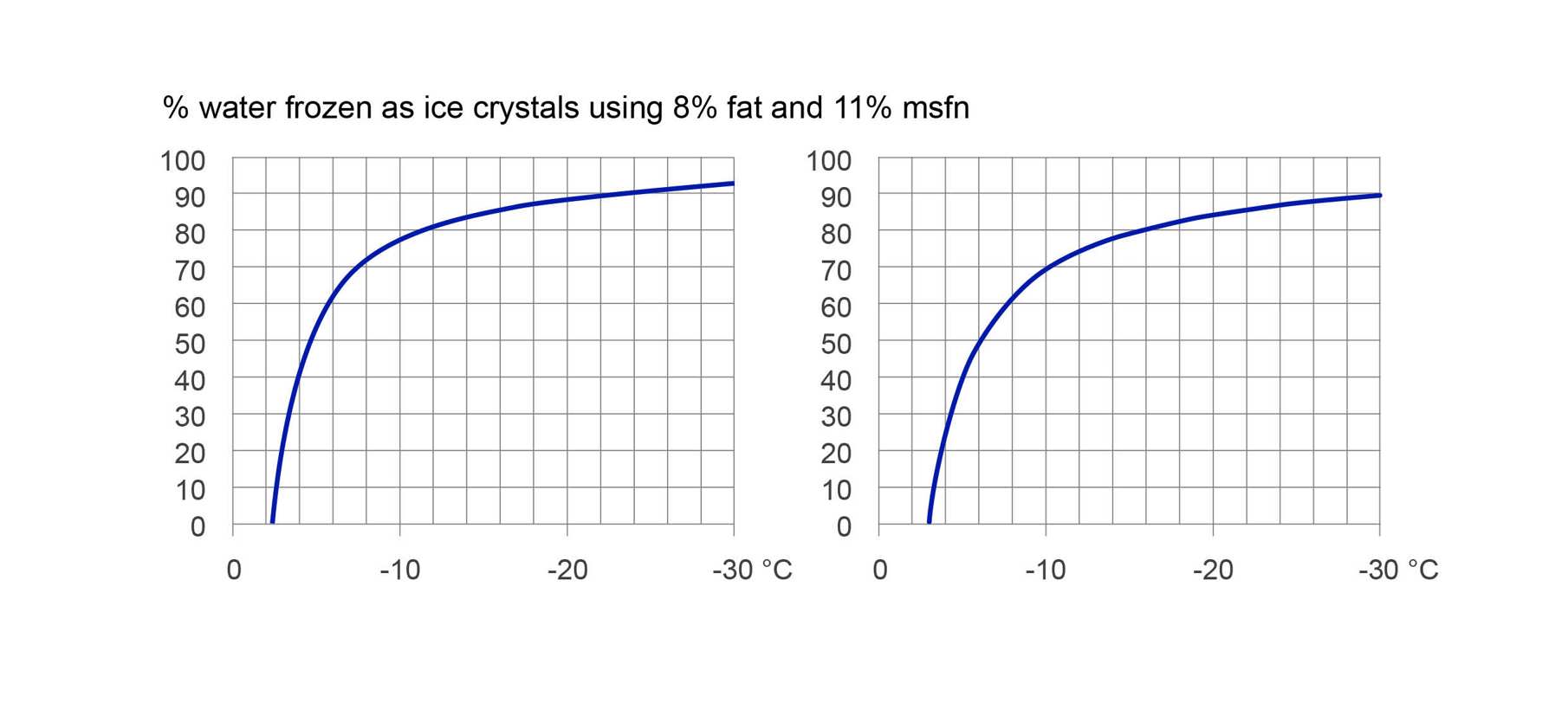
Even with the same fat and milk solid content, the percentage of water frozen into ice crystals will vary if sugar levels or types are adjusted.
Figure 2 shows the effect of a different sugar composition alone on the freezing curve of ice cream containing 8% fat and 11% milk-solids-nonfat (MSNF).
Sugar, of course, comes in different types. Ice creams typically use a combination of refined sugar and, for example, glucose syrup. This combination affects the texture and the freezing point of the product, too. Some producers even add a little salt to the mix, further depressing the freezing point and adding to heat shock susceptibility.
Luckily (or rather, the result of over 100 years of development), modern, plant-based emulsifiers are available that can go a lot of the way toward protecting ice creams from heat shock.
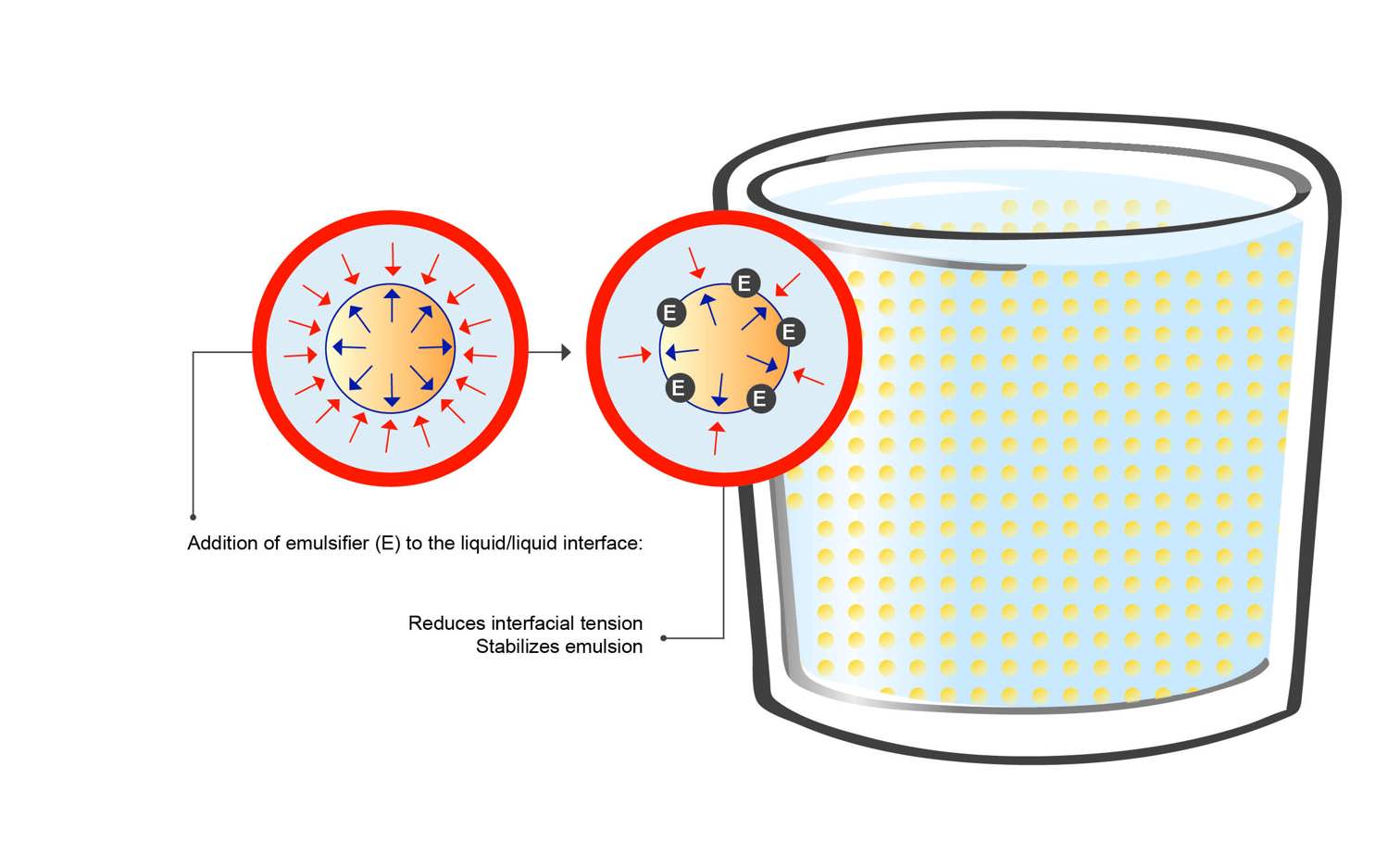
As Figure 3 visualises, emulsifiers are surface-active ingredients due to their hydrophilic-lipophilic properties, placing themselves in the interfacial layer between the fat/protein and water and helping to improve or control:
The most common emulsifiers used in ice cream are mono-diglycerides (E471), lactic acid esters (E472b), propylene glycol esters (E477) and blends of these.
Generally speaking, good compositions can be made with most of the widely-used emulsifiers and stabilisers such as mono- and diglycerides, carrageenan and locust bean gum. If that is, they are used in the right amount and compositions to suit each recipe.
Adding propylene glycol esters such as propylene glycol monostearate (PGMS), for example, protects against the Heat Shock Effect by ensuring small ice crystals are created during freezing and reducing their tendency to grow during the journey to the consumer’s table, as shown in Figure 4.
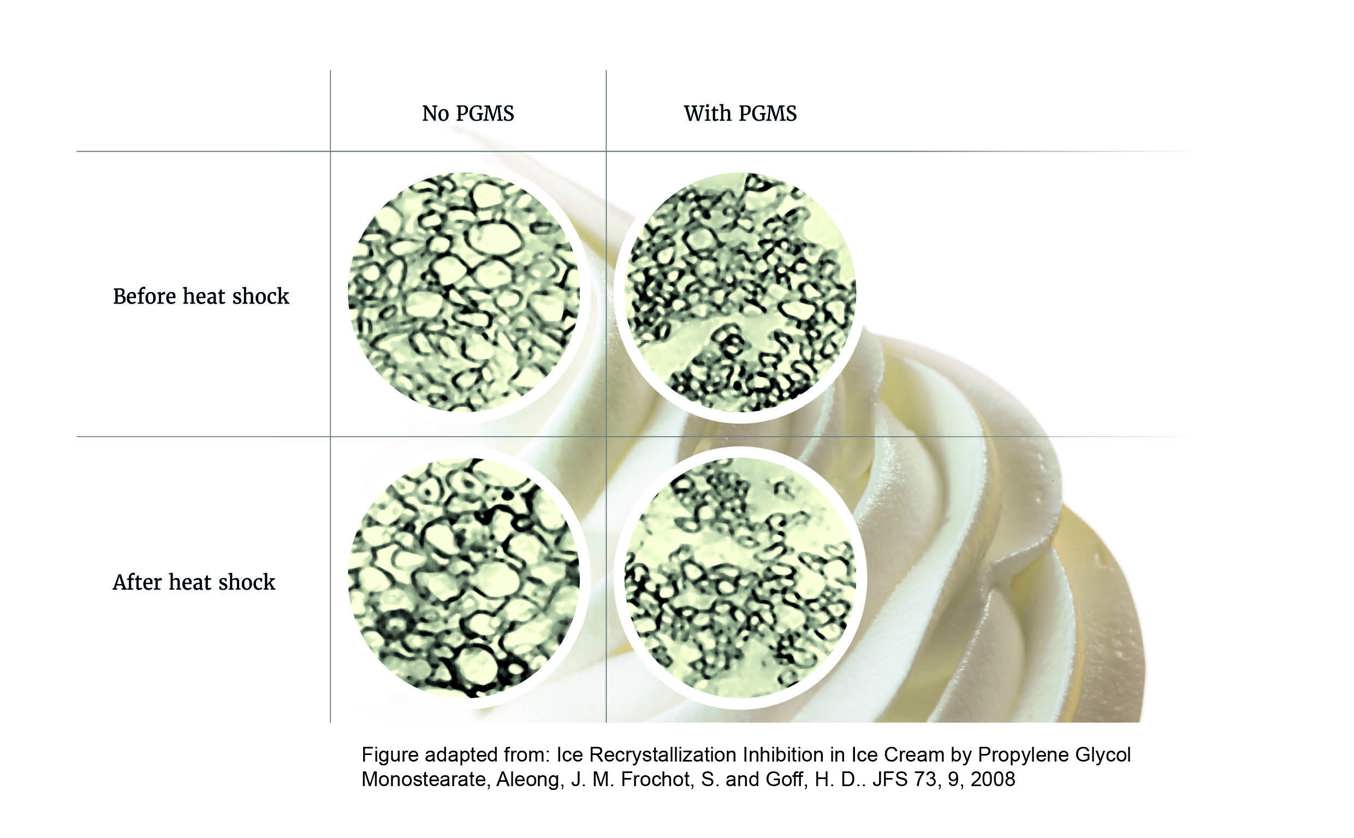
During the freezing stage of ice cream production, the goal is to create as many small ice crystals as possible, providing as large a total surface area as possible.
The freezer process, which also includes whipping, doesn’t freeze all the water, as the ice cream leaves the freezer at around -6 °C (21.2 °F) ready for filling in tubs or placing onto sticks. As soon as this is done, and to provide the best starting point for heat shock protection, the product needs to be placed as quickly as possible into a hardening tunnel at -40°C (-40 °F) and then finally in the storage freezer at -18°C (-0.4°F).
Unfortunately, control is not always strict enough in this stage of production, with some plants transporting the ice cream via long pipes where the temperature may raise, for example, to -3 °C (26.6 °F) before filling begins. The filling process elevates the temperature further, then there is likely to be waiting time on a pallet, too, until the pallet is full and can be placed in appropriately cold storage. In warm climates or seasons, this can have quite an effect, with some of the ice crystals already starting to melt, turning into water and becoming part of the growth of other crystals. The longer it takes to deep-freeze the entire product, the more water will move in this way and the greater early heat shock damage will be.
Attention also needs to be paid to ageing, the stage that takes place between mixing and freezing steps, requiring pasteurization to dissolve the emulsifiers and stabilisers and kill off bacteria, as well as homogenization to support the fat structure and cooling at -5 °C (23 °F) for anything from 4 hours to overnight.

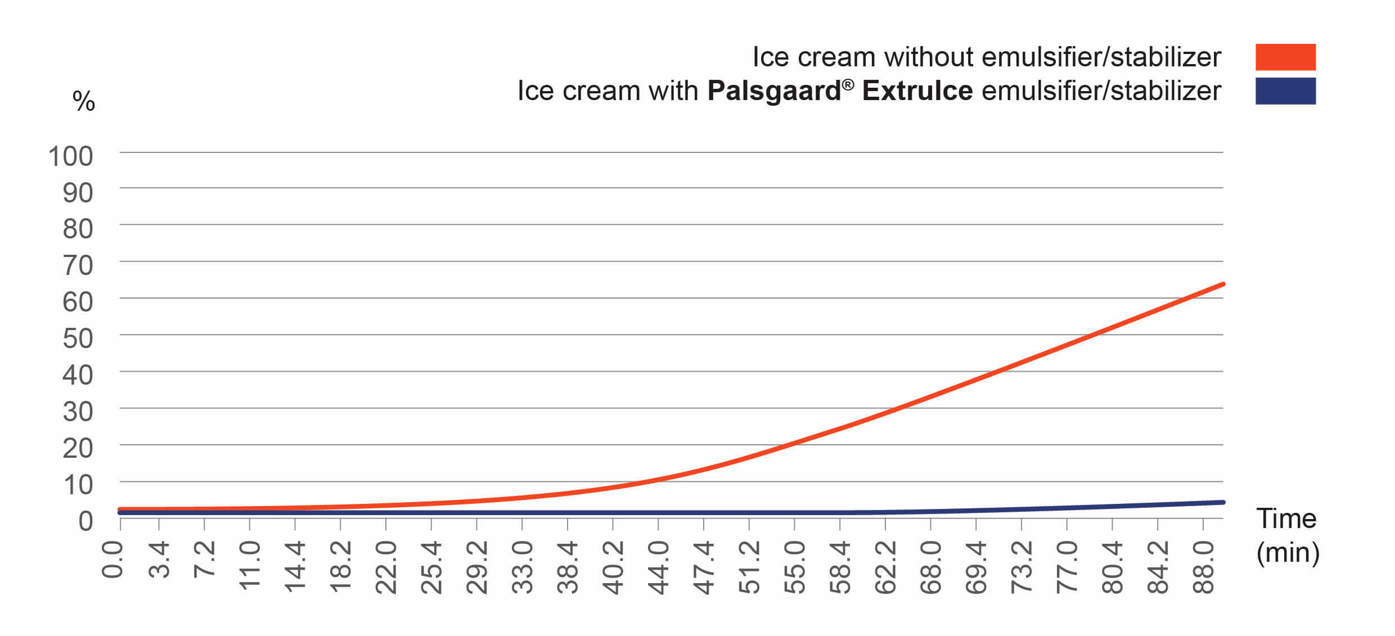
Figure 5 depicts two melting curves of an ice cream that was melted using a special machine for accurate measurement. The amount of ice cream landing in bowls placed under the product was measured as a percentage of its overall weight. The upper curve reflects an ordinary ice cream recipe, while the lower curve, which performs far better, shows the effect of adding one of Palsgaard’s integrated blends of emulsifiers and stabilisers, Palsgaard® ExtruIce.
It’s worth noting that melting curves can be influenced by homogenization, too, if homogenisation isn’t conducted at the right pressure level. This doesn’t directly affect heat shock robustness, as such, but may weaken the ice cream’s structure and hasten melting.
So, which emulsifier is best for countering the effects of heat shock? Monodiglyceride (E471), for example, comes in many forms and qualities (differing in fatty acid composition, and in the levels of mono and diglycerides) each bringing out different qualities in ice cream.
Composing an emulsifier stabiliser blend with the right composition to work with an ice cream’s structure, protect against heat shock and achieve everything else the manufacturer and the product’s consumers would like takes a great deal of expertise. And it demands an unrelenting eye on the Big Picture, comprising ingredients, emulsifier/stabiliser composition, process and equipment. You may also be wise to discuss your product’s packaging with its manufacturer, who may be able to recommend better packaging and handling solutions.
At Palsgaard, we work with different recipes to find the right combination and quality of emulsifiers and stabilisers. We know, for example, that not all emulsifiers will work well with milk replacers (cheaper milk solids used to replace skim milk powders) and they’re likely to have a different effect depending on the type of milk solids used, too. And that means there is much more trial and error involved in figuring out exactly which additives, combinations of additives and dosages will work best.
What is your heat shock protection strategy? It’s likely to depend on factors such as your product’s market positioning in terms of quality, conditions at your factory and the realities of price points, for example, in your part of the world.
Whichever steps you take, you can be sure that building heat shock stability into your ice creams will help to maintain their quality, providing a better consumer experience and a more secure future for your products in retail outlets. Without a doubt, the emulsifier/stabiliser blends you choose will be at the heart of making it all happen.
Reviewing your emulsifier choices offers the chance to introduce ingredients sourced and produced under recognised sustainability standards, meeting growing consumer and regulatory expectations. For example, Palsgaard’s range of non-GMO, plant-based emulsifiers is manufactured in facilities with a strategic focus on reducing energy consumption and CO₂ emissions. In addition, we offer our palm oil-based emulsifiers as RSPO Segregated (SG) certified options, supporting traceability and responsible practices throughout the supply chain.

TROUBLESHOOTING BY PALSGAARD

TECHNICAL ARTICLE

TECHNICAL ARTICLE

TECHNICAL ARTICLE

TECHNICAL ARTICLE

TECHNICAL ARTICLE

TECHNICAL ARTICLE

TECHNICAL ARTICLE